Experimental animals with the highest degree of suffering in 2021
The Danish 3R-Center, June 2022
For several years, the Danish 3R-Center has been focused on the experimental animals that experience severe suffering as the result of experimental procedures. The reason for this has been for the 3R-Center to remain updated on the number of animals used in these procedures and to discuss the possibilities for reducing the amount of suffering inflicted on the animals.
Due to the EU Directive on the protection of animals used for scientific purposes, animal experiments must be classified according to the degree of suffering that the animals experience during the experiment (mild, moderate or severe). Animals that are anesthetized during an experiment and do not recover consciousness afterwards as a result of the procedure(s) are categorised as non-recovery.
The severe category represents the group of animals that experience the most severe degree of suffering in regards to what is allowed by the Animal Experiments Act. The number and type of animals inflicted with the highest degree of suffering can be seen in Table 1.1
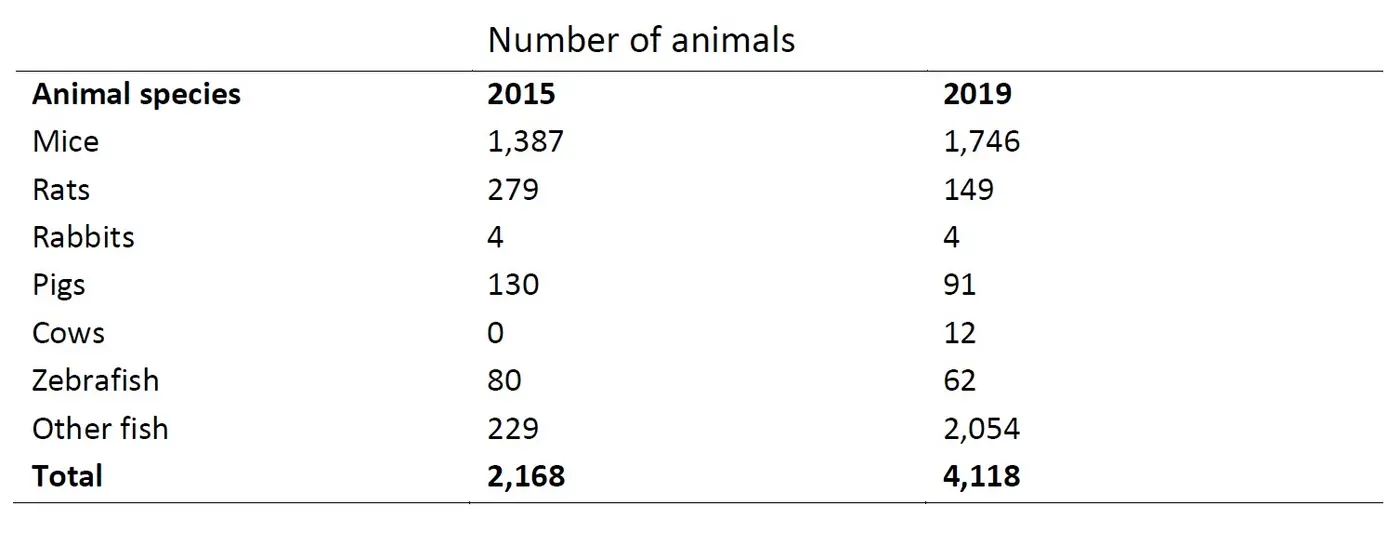
These interviews took place on the basis of the number of experimental animals used in 2015 and 2019.2
The interviews were meant to investigate why it is considered necessary to inflict severe suffering on animals and if it is possible to replace these experiments with experiments that cause less severe suffering or animal-free models.
According to the Animal Experiments Act, when an animal experiment application is received by the Animal Experimentation Inspectorate, the Inspectorate must discuss the level of expected suffering inflicted on the animals in regards to the scientific benefits of the research. The Danish 3R-Center has thus held the interviews on the basis that there must have been well-founded and law-abiding reason(s) for the Inspectorate to grant permission for the interviewee’s experiments.
It must be noted that the interviews found that the researchers reported very thorough case management with a lot of dialogue prior to obtaining the animal experimentation permit from the Inspectorate.
The number of animals inflicted with severe suffering increased from 2015 to 2019 (see Table 1). This increase is mostly due to a substantial increase of the number of fishes that were categorized as having experienced severe suffering.
The results may not be entirely comparable due to one of the results from the 2015 interviews. The interviews found that that there had been confusion regarding the categorization of animal experiments due to new Directive provisions. This confusion led to too many animals being reported and categorized as having experienced severe suffering in 2015, because some of the researchers chose to report that all their animals had experienced severe suffering despite the fact that some individual animals experienced a less severe category of suffering. The EU-Directive states that the inflicted suffering on each animal must be individually evaluated. However, it cannot be assumed that the increase in the number of animals reported in the severe category is due to this uncertainty, and therefore, the increase must be considered to be legit.
The 2021 interviews found a strong decrease in the number of severe reported animals, but also found that this decrease was likely because of the corona lock-downs. The decrease number of animals is therefore expected to be a temporary occurrence.
The use of animals in the severe category is can be categorised into two subgroups:
- Disease models for especially painful conditions in humans
- Statutory investigations with unknown endpoints
Disease models for especially painful conditions in humans
Health science research and drug development includes inducing diseases and/or disorders in experimental animals so that researchers can study the disease development, methods of treatment, etc. In Denmark, disabling neurological and psychiatric diseases are mostly studied, as well as intestinal diseases in premature babies. The most used animal species for studying neurological and psychiatric diseases are mice and the most used species for studying intestinal diseases are pigs.
Some diseases can be particularly painful for patients, and when these diseases are induced in animals, the animals will experience similar suffering. Due to the extensive interviews, it is the Danish 3R-Center belief that a large number of in vitro and in vivo methods are used before the painful diseases are induced in animals. The use of in vitro and in vivo methods aids in reducing the need for experiments that inflict severe suffering on animals. However, the 3R-Center also notes that it may, in some circumstances, be possible to obtain an earlier and more humane endpoint for the animals if monitoring is intensified and refined. This refinement of monitoring methods requires development.
Mice are often used as a model for multiple sclerosis and constitute a large part of this group of disease models. They are referred to as the EAE-models. AEA stands for Experimental Autoimmune Encephalitis, and AEA is induced in mice by treating them with a substance that can be found around nerve cells. After a mouse is treated with this substance, its immune system will cause an immune response that attacks the mouse’s nervous system. This response will cause a paralysis in the mouse that starts in the forelegs and gradually moves to the hindlegs before it decreases altogether. EAE is not painful per se, but the Animal Experimentation Inspectorate does categorize EAE-model animals as severe suffering due to the progressive paralysis.
The Danish 3R-Center has noticed that not all research groups apply for permits with the same level of suffering for this model. The opportunity to develop a way for this model to inflict a lower category of suffering exists, but it requires researchers to take advantage of each other’s experience. Therefore, the 3R-Center would like to develop a network of these research groups, so that researchers can share ideas and discuss if it is possible to achieve an acceptable research yield while inflicting a lower category of suffering on the animals.
Statutory investigations with unknown endpoints
International regulations (EU-directives, drug registration requirements for non-EU countries, etc.) can result in Denmark being obligated to undertake research where the effect(s) of the experiment is unknown. This may cause the animals to experience severe suffering.
New substances that could potentially end up in our water-systems must be tested to see if they can cause disease and death in animals in nature. These experiments are mostly conducted on fish (included in the Animal Experimentation Act) and crustaceans (not included in the Animal Experimentation Act). It can be difficult to identify early symptoms of disease or injury in fish and this may result some fish dying during such experiments if the substances are found to have harmful effect.
This is supported by the requirement of positive control groups in such experiments. A positive control groups means that a separate experiment with fish receiving a drug with known effects must take place simultaneously as the experiment of the substance with unknown effects. Many tested unknown substances turnout to be harmless, so most of the fish that experience severe suffering can be found in the positive control groups.
Clarification of the unknown infections in fish requires inoculation of the fish under experimental conditions. Symptoms can develop quickly during this time and are difficult to identify before the fish dies. There has been a significant increase in the use of animals in such experiments since 2015, which may be the result of Denmark becoming a reference laboratory.
There are also varying statutory requirements for testing the toxic effect(s) of chemical substances and the side effect(s) of medicinal drugs, which may result in animals experiencing the highest degree of suffering. These experiments are often performed on rats. Medicinal drugs are typically researched with in vitro and in vivo methods during their long development phase. These studies will rarely result in violent and acute effects as opposed to chemical substances, which are often not tested as much as medicinal drugs during their toxicological testing path.
The 3R-Center believes that there may be reason(s) to consider if the experimental designs(s) used in statutory investigations are the most appropriate from a 3R point of view. For example: it may be more prudent to investigate if all the positive control groups are needed in order to draw meaningful conclusions.
This is not something that can be regulated by the consumers, the Animal Experiment Inspectorate or the Danish Parliament. Most of the statutory requirements are derived from international regulations, and future improvements to the field are therefore very dependent on Danish agencies, such as the Danish Medicine agency, the Danish Environmental Protection
Agency and the Danish Veterinary and Food Administration. Therefore, the 3R-Center will strive to maintain an ongoing dialogue with these agencies in order to ensure that all sides will remain well-informed.
Furthermore, the 3R-Center has found that researchers that use fish in their studies have different methods of reducing the use of fish the ‘severe’ category. The establishment of research groups for these researchers has been considered, so that they may draw on each other’s experience and ideas.
Conclusion
There is no reason to assume that Denmark’s use of animals categorised with being inflicted with the highest degree of suffering will significantly decrease in the next few years. However, the researcher interviews indicate that the approval of severe experiments is a lengthy and thorough process in the Animal Experimentation Inspectorate.
The Danish 3R-Center finds it likely that creating research groups for researchers that use the same animal species would allow the researchers to draw on each other’s experience. The 3R-Center also emphasises the importance of ensuring an ongoing dialogue with Danish agencies who can influence international decisions about animal research.
Furthermore, the 3R-Center also finds it may be relevant for some researchers to apply for funding from the Danish 3R-Center or other centres. This funding can be used to develop initiatives that can be used to reduce the suffering of these animals. However, the Danish 3R-Center has limited research funds available and all initiatives are therefore in competition with other 3R-initiatives.
1 The table does not include animals that died spontaneously without a diagnosis that ruled out severe suffering and was therefore recorded as having experience severe suffering. In 2019, this concerned 228 animals. This number describes the number of spontaneous fatalities amongst experimental animals, and it is not a number of concern when the total number of used experimental animals is considered.
2 In 2017 and 2021, the 3R-Center’s interview committee consisted of the following Board members: Lisbeth E. Knudsen, Jan Lund Ottesen, and Axel Kornerup Hansen.
Newsletter
Sign up for our newsletter - don't miss information about our annual symposium etc.